what is the hardest thing in the world to cut
A superhard cloth is a textile with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test.[1] [2] [3] [iv] They are nigh incompressible solids with high electron density and high bond covalency. Equally a result of their unique properties, these materials are of bang-up interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.
Diamond is the hardest known cloth to date, with a Vickers hardness in the range of 70–150 GPa. Diamond demonstrates both loftier thermal conductivity and electrically insulating properties, and much attention has been put into finding applied applications of this material. However, diamond has several limitations for mass industrial application, including its high price and oxidation at temperatures above 800 °C.[v] [6] In add-on, diamond dissolves in fe and forms iron carbides at loftier temperatures and therefore is inefficient in cut ferrous materials including steel. Therefore, recent research of superhard materials has been focusing on compounds which would exist thermally and chemically more stable than pure diamond.
The search for new superhard materials has generally taken two paths.[seven] In the first arroyo, researchers emulate the curt, directional covalent carbon bonds of diamond by combining light elements like boron, carbon, nitrogen, and oxygen. This approach became popular in the belatedly 1980s with the exploration of C3Due north4 and B-C-N ternary compounds. The 2d approach towards designing superhard materials incorporates these lighter elements (B, C, N, and O), but likewise introduces transition metals with high valence electron densities to provide loftier incompressibility. In this manner, metals with high majority moduli but low hardness are coordinated with small covalent-forming atoms to produce superhard materials. Tungsten carbide is an industrially-relevant manifestation of this approach, although it is not considered superhard. Alternatively, borides combined with transition metals have go a rich surface area of superhard research and have led to discoveries such as ReB2, OsB2, and WB4.
Superhard materials tin be more often than not classified into two categories: intrinsic compounds and extrinsic compounds. The intrinsic grouping includes diamond, cubic boron nitride (c-BN), carbon nitrides, and ternary compounds such every bit B-N-C, which possess an innate hardness. Conversely, extrinsic materials are those that have superhardness and other mechanical backdrop that are adamant by their microstructure rather than composition.[8] [9] [ten] An example of extrinsic superhard material is nanocrystalline diamond known as aggregated diamond nanorods.

A nanoindenter, used to measure the hardness and related properties of materials
Definition and mechanics of hardness [edit]

An indentation left in example-hardened steel after a Vickers hardness test.
The hardness of a material is directly related to its incompressibility, elasticity and resistance to change in shape. A superhard fabric has loftier shear modulus, high bulk modulus, and does not deform plastically. Ideally superhard materials should have a defect-free, isotropic lattice. This profoundly reduces structural deformations that can lower the strength of the material. Nevertheless, defects can actually strengthen some covalent structures. Traditionally, high-force per unit area and high-temperature (HPHT) conditions take been used to synthesize superhard materials, but recent superhard fabric syntheses aim at using less energy and lower price materials.[9] [x]
Historically, hardness was offset defined as the power of ane textile to scratch some other and quantified by an integer (sometimes half-integer) from 0 to x on the Mohs calibration. This scale was nonetheless quickly constitute too discrete and non-linear. Measuring the mechanical hardness of materials changed to using a nanoindenter (usually made of diamond) and evaluating bulk moduli, and the Brinell, Rockwell, Knoop, and Vickers scales have been developed. Whereas the Vickers scale is widely accepted as a nearly common test,[ten] there remain controversies on the weight load to exist applied during the test. This is considering Vickers hardness values are load-dependent. An indent fabricated with 0.5N will indicate a higher hardness value than an indent made with 50N. This phenomenon is known as the indentation size effect (ISE). Thus, hardness values are not meaningful unless the load is likewise reported. Some fence that hardness values should consistently be reported in the asymptotic (high-load region), every bit this is a more than standardized representation of a textile's hardness.[xi]
| Cloth | Vickers hardness (GPa) | Bulk Modulus (GPa) |
|---|---|---|
| Diamond | 115[12] | 440 |
| c-BC2N | 76 | 282 |
| c-BCv | 71[12] | |
| γ-Boron | 58 | 227 |
| c-BN | 48, 62[12] | 400 |
| OsB2 | 37 | 395 |
| B4C | 35, 38[12] | |
| WB4 | ~30 | |
| AlMgB14 | 26.vii[14] | |
| ReBtwo | ~20 |
Bulk moduli, shear moduli, and elasticity are the fundamental factors in the superhard classification process. The incompressibility of a fabric is quantified by the bulk modulus B, which measures the resistance of a solid to volume compression under hydrostatic stress as B = −Vdp/dV. Here V is the volume, p is force per unit area, and dp/dV is the partial derivative of pressure with respect to the volume. The bulk modulus test uses an indenter tool to form a permanent[ dubious ] deformation in a textile.[ commendation needed ] The size of the deformation depends on the material's resistance to the book pinch fabricated by the tool. Elements with small molar volumes and potent interatomic forces normally have loftier bulk moduli. Bulk moduli was the first major examination of hardness and originally shown to be correlated with the molar volume (5m) and cohesive energy (Ec) as B ~ Ec/5thou.
Bulk modulus was believed to be a direct measure of a material's hardness just this no longer remains the dominant school of thought. For example, some alkali and noble metals (Pd, Ag) take anomalously high ratio of the bulk modulus to the Vickers of Brinell hardness. In the early on 2000s, a direct relationship between bulk modulus and valence electron density was found as the more electrons were present the greater the repulsions within the structure were.[9] Majority modulus is nevertheless used as a preliminary measure of a material as superhard but it is now known that other backdrop must be taken into account.[9] [10]
In contrast to bulk modulus, shear modulus measures the resistance to shape alter at a abiding volume, taking into business relationship the crystalline plane and direction of shear. The shear modulus G is defined equally ratio of shear stress to shear strain: G = stress/strain = F·50/(A·dx), where F is the applied force, A is the area upon which the force acts, dx is the resulting displacement and L is the initial length. The larger the shear modulus, the greater the ability for a fabric to resist shearing forces. Therefore, the shear modulus is a measure of rigidity. Shear modulus is related to bulk modulus as 3/Thousand = 2B(one − 2v)(i + five), where v is the Poisson's ratio, which is typically ~0.1 in covalent materials. If a material contains highly directional bonds, the shear modulus will increase and give a depression Poisson ratio.
A material is as well considered difficult if it resists plastic deformation. If a material has short covalent bonds, diminutive dislocations that lead to plastic deformation are less probable to occur than in materials with longer, delocalized bonds. If a material contains many delocalized bonds it is likely to exist soft.[9] Somewhat related to hardness is some other mechanical holding fracture toughness, which is a cloth'south power to resist breakage from forceful impact (notation that this concept is singled-out from the notion of toughness). A superhard material is not necessarily "supertough". For example, the fracture toughness of diamond is about vii–10 MPa·yard1/2,[fifteen] [xvi] which is high compared to other gemstones and ceramic materials, just poor compared to many metals and alloys – mutual steels and aluminium alloys accept the toughness values at least v times higher.[17]
Several properties must exist taken into account when evaluating a textile equally (super)difficult. While difficult materials accept high bulk moduli, a loftier majority modulus does non mean a material is hard. Inelastic characteristics must be considered every bit well, and shear modulus might even provide a better correlation with hardness than bulk modulus. Covalent materials generally have high bond-bending force constants and loftier shear moduli and are more than likely to give superhard structures than, for case, ionic solids.[ix] [ten]
Diamond [edit]

Diamond and graphite materials and construction
Diamond is an allotrope of carbon where the atoms are arranged in a modified version of face-centered cubic (fcc) structure known as "diamond cubic". It is known for its hardness (see table above) and incompressibility and is targeted for some potential optical and electrical applications. The properties of individual natural diamonds or carbonado vary too widely for industrial purposes, and therefore synthetic diamond became a major research focus.[eighteen] [19]
Synthetic diamond [edit]
The loftier-pressure synthesis of diamond in 1953 in Sweden[20] [21] and in 1954 in the U.s.,[22] made possible past the development of new apparatus and techniques, became a milestone in synthesis of artificial superhard materials. The synthesis clearly showed the potential of high-pressure applications for industrial purposes and stimulated growing involvement in the field. Four years afterward the commencement synthesis of artificial diamond, cubic boron nitride c-BN was obtained and plant to exist the 2d hardest solid.[23]
Synthetic diamond can exist equally a single, continuous crystal or as small polycrystals interconnected through the grain boundaries. The inherent spatial separation of these subunits causes the formation of grains, which are visible past the unaided eye due to the lite absorption and scattering properties of the material.[24]
The hardness of constructed diamond (seventy–150 GPa) is very dependent on the relative purity of the crystal itself. The more perfect the crystal construction, the harder the diamond becomes. It has been reported that HPHT single crystals and nanocrystalline diamond aggregates (aggregated diamond nanorods) tin be harder than natural diamond.[24]
Historically, it was thought that synthetic diamond should be structurally perfect to be useful. This is because diamond was mainly preferred for its artful qualities, and pocket-sized flaws in structure and limerick were visible by naked eye. Although this is truthful, the properties associated with these small changes has led to interesting new potential applications of synthetic diamond. For example, nitrogen doping can enhance mechanical forcefulness of diamond,[25] and heavy doping with boron (several atomic percent) makes it a superconductor.[26]
In 2014, researchers reported on the synthesis of nano-twinned[ clarification needed ] diamond with Vickers hardness values upwardly to 200 GPa.[27] The authors attribute the unprecedented hardness to the Hall-Petch issue, which predicts that smaller microstructural features can lead to enhanced hardness due to higher density of boundaries that stop dislocations. They achieve twins with an average thickness of 5 nm using a precursor of onion[ clarification needed ] carbon nanoparticles subjected to high temperature and pressure. They too simultaneously accomplish an oxidation temperature that is 200 °C higher than that of natural diamond. Higher thermal stability is relevant to industrial applications such as cutting tools, where high temperatures tin lead to rapid diamond degradation.
Dumbo Amorphous Carbon [edit]
A dense AM-3 form of transparent amorphous carbon has a Vickers hardness of 113 GPa.[28] This heat-treated fullerene is currently the hardest amorphous material.
Cubic boron nitride [edit]
History [edit]
Cubic boron nitride or c-BN was get-go synthesized in 1957 by Robert H. Wentorf at General Electric, shortly later on the synthesis of diamond.[23] The full general process for c-BN synthesis is the dissolution of hexagonal boron nitride (h-BN) in a solvent-catalyst, usually alkali or alkali metal earth metals or their nitrides, followed by spontaneous nucleation of c-BN under high pressure, high temperature (HPHT) weather condition.[10] The yield of c-BN is lower and substantially slower compared to diamond's synthetic route due to the complicated intermediate steps. Its insolubility in fe and other metal alloys makes it more useful for some industrial applications than diamond.[29]

Pure cubic boron nitride is transparent or slightly amber. Different colors tin can exist produced depending on defects or an excess of boron (less than 1%).[x] Defects tin be produced past doping solvent-catalysts (i.e. Li, Ca, or Mg nitrides) with Al, B, Ti, or Si. This induces a change in the morphology and colour of c-BN crystals.[thirty] The result is darker and larger (500 μm) crystals with better shapes and a higher yield.
Structure and properties [edit]
Cubic boron nitride adopts a sphalerite crystal construction, which can be constructed past replacing every two carbon atoms in diamond with i boron cantlet and one nitrogen atom. The curt B-N (1.57 Å) bond is close to the diamond C-C bond length (1.54 Å), that results in strong covalent bonding betwixt atoms in the same fashion as in diamond. The slight subtract in covalency for B-Due north bonds compared to C-C bonds reduces the hardness from ~100 GPa for diamond downward to 48 GPa in c-BN. As diamond is less stable than graphite, c-BN is less stable than h-BN, but the conversion rate between those forms is negligible at room temperature.[29]
Cubic boron nitride is insoluble in iron, nickel, and related alloys at high temperatures, but it binds well with metals due to formation of interlayers of metal borides and nitrides. It is also insoluble in most acids, but is soluble in element of group i molten salts and nitrides, such as LiOH, KOH, NaOH/Na2CO3, NaNO3 which are used to etch c-BN.[31] Because of its stability with heat and metals, c-BN surpasses diamond in mechanical applications. The thermal conductivity of BN is among the highest of all electric insulators. In add-on, c-BN consists of only lite elements and has low X-ray absorptivity, capable of reducing the X-ray absorption background.[32]
Research and development [edit]
Due to its corking chemical and mechanical robustness, c-BN has widespread application equally an annoying, such as on cut tools and scratch resistant surfaces. Cubic boron nitride is besides highly transparent to X-rays. This, along with its loftier strength, makes it possible to take very thin coatings of c-BN on structures that can be inspected using Ten-rays. Several hundred tonnes of c-BN are produced worldwide each year.[33] By modification, Borazon, a United states brand name of c-BN, is used in industrial applications to shape tools, as it can withstand temperatures greater than 2,000 °C. Cubic boron nitride-coated grinding wheels, referred to equally Borazon wheels, are routinely used in the machining of difficult ferrous metals, bandage irons, and nickel-base and cobalt-base of operations superalloys. Other make names, such as Elbor and Cubonite, are marketed by Russian vendors.[29]
New approaches in inquiry focus on improving c-BN pressure level capabilities of the devices used for c-BN synthesis.[10] At present, the capabilities for the production of c-BN are restricted to pressures of nigh 6 GPa. Increasing the pressure limit will permit synthesis of larger single crystals than from the present catalytic synthesis. All the same, the use of solvents under supercritical weather for c-BN synthesis has been shown to reduce force per unit area requirements.[ten] The loftier cost of c-BN still limits its application, which motivates the search for other superhard materials.
Carbon nitride [edit]
The structure of beta carbon nitride (β-C3Niv) was first proposed past Amy Liu and Marvin Cohen in 1989. It is isostructural with Si3N4 and was predicted to exist harder than diamond.[34] The calculated bond length was 1.47 Å, five% shorter than the C-C bond length in diamond. Later calculations indicated that the shear modulus is 60% of that of diamond, and carbon nitride is less difficult than c-BN.[35]
Despite two decades of pursuit of this chemical compound, no synthetic sample of C3N4 has validated the hardness predictions; this has been attributed to the difficulty in synthesis and the instability of C3N4. Carbon nitride is only stable at a pressure that is higher than that of the graphite-to-diamond transformation. The synthesis conditions would crave extremely loftier pressures considering carbon is four- and sixfold coordinated.[10] In add-on, CthreeN4 would pose bug of carbide formation if they were to exist used to machine ferrous metals. Although publications have reported preparation of C3N4 at lower pressures than stated, synthetic C3Niv was not proved superhard.[36]
Boron carbon nitride [edit]
The similar diminutive sizes of boron, carbon and nitrogen, also as the similar structures of carbon and boron nitride polymorphs, suggest that it might exist possible to synthesize diamond-similar stage containing all iii elements. It is as well possible to make compounds containing B-C-O, B-O-N, or B-C-O-Due north under loftier pressure level, but their synthesis would expect to crave a complex chemistry and in addition, their elastic properties would be inferior to that of diamond.
First in 1990, a smashing interest has been put in studying the possibility to synthesize dense B-C-Due north phases. They are expected to be thermally and chemically more stable than diamond, and harder than c-BN, and would therefore exist excellent materials for loftier speed cutting and polishing of ferrous alloys. These characteristic properties are attributed to the diamond-like structure combined with the sp3 σ-bonds amidst carbon and the heteroatoms. BC10Due northy thin films were synthesized past chemical vapor deposition in 1972.[37] Nevertheless, data on the attempted synthesis of B-C-North dense phases reported by dissimilar authors have been contradictory. It is unclear whether the synthesis products are diamond-like solid solutions between carbon and boron nitride or just mechanical mixtures of highly dispersed diamond and c-BN. In 2001, a diamond-like-structured c-BC2N was synthesized at pressures >eighteen GPa and temperatures >ii,200 K by a direct solid-country phase transition of graphite-like (BN)0.48C0.52. The reported Vickers and Knoop hardnesses were intermediate between diamond and c-BN, making the new phase the second hardest known material.[38] Ternary B–C–N phases tin can likewise be made using shock-compression synthesis. Information technology was farther suggested to extend the B–C–Due north system to quaternary compounds with silicon included.[8] [39]
Metal borides [edit]
Unlike carbon-based systems, metallic borides tin can be easily synthesized in large quantities under ambient conditions, an important technological advantage.[ix] Nigh metal borides are difficult;[xl] yet, a few stand out among them for their specially high hardnesses (for example, WBiv,[41] [42] RuB2, OsB2 and ReB2). These metallic borides are notwithstanding metals and not semiconductors or insulators (equally indicated by their loftier electronic density of states at the Fermi Level); however, the boosted covalent B-B and M-B bonding (M = metal) lead to high hardness.[43] [44] Dense heavy metals, such as osmium, rhenium, tungsten etc., are particularly apt at forming hard borides considering of their loftier electron densities, small atomic radii, high bulk moduli, and ability to bond strongly with boron.
Osmium diboride [edit]

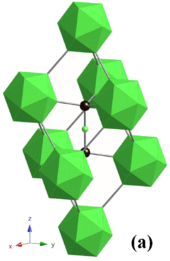
Crystal structure of OsB2
Osmium diboride (OsB2) has a loftier majority modulus of 395 GPa and therefore is considered as a candidate superhard fabric, but the maximum accomplished Vickers hardness is 37 GPa, slightly below the 40 GPa limit of superhardness. A mutual way to synthesize OsB2 is past a solid-state metathesis reaction containing a 2:3 mixture of OsCl3:MgBtwo.[9] After the MgCltwo product is washed away, Ten-ray diffraction indicates products of OsBtwo, OsB and Os. Heating this product at ane,000 °C for three days produces pure OsB2 crystalline product. OsB2 has an orthorhombic structure (infinite group Pmmn) with two planes of osmium atoms separated by a non-planar layer of hexagonally coordinated boron atoms; the lattice parameters are a = four.684 Å, b = two.872 Å and c = iv.096 Å.[9] The b direction of the crystal is the virtually compressible and the c direction is the least compressible.[45] This can exist explained by the orthorhombic construction. When looking at the boron and osmium atoms in the a and b directions, they are bundled in a way that is offset from one another. Therefore, when they are compressed they are non pushed correct up against ane another. Electrostatic repulsion is the force that maximizes the materials incompressibility and so in this case the electrostatic repulsion is not taken total advantage of. When compressed in the c management, the osmium and boron atoms are almost directly in line with one another and the electrostatic repulsion is therefore loftier, causing direction c to exist the least compressible. This model implies that if boron is more evenly distributed throughout the lattice then incompressibility could be higher. Electron backscatter diffraction coupled with hardness measurements reveals that in the (010) aeroplane, the crystal is 54% harder in the <100> than <001> direction. This is seen past looking at how long the indentation is forth a sure direction (related to the indentations made with a Vickers hardness test). Forth with the alignment of the atoms, this is likewise due to the brusk covalent B-B (1.lxxx Å) bonds in the <100> direction, which are absent in the <001> management (B-B = 4.10 Å).[9]
Rhenium borides [edit]
Rhenium was targeted every bit a candidate for superhard metal borides considering of its desirable physical and chemical characteristics. It has a high electron density, a modest atomic radius and a high bulk modulus. When combined with boron, it makes a crystal with highly covalent bonding allowing it to be incompressible and potentially very difficult.[46] A wide assortment of rhenium borides have been investigated including Re3B, Re7B3, Re2B, ReB, Re2Biii, Re3B7, Re2B5, ReB3 and ReB2. Each of these materials has their own ready of backdrop and characteristics. Some bear witness promise every bit superconductors and some have unique elastic and electronic properties, but the near relevant to superhard materials is ReB2.[46]
Rhenium diboride (ReBii) is a refractory compound which was first synthesized in the 1960s, using arc melting, zone melting, or optical floating zone furnaces. An example synthesis of this fabric is the flux method, which is conducted by placing rhenium metal and amorphous boron in an alumina crucible with excess aluminium. This can exist run with a ratio of ane:two:50 for Re:B:Al, with the excess aluminium as a growth medium. The crucible is placed in an alumina tube, inserted into a resistively heated furnace with flowing argon gas and sintered at 1,400 °C for several hours. Later on cooling, the aluminium is dissolved in NaOH. Each ReB2 synthesis route has its own drawbacks, and this one gives modest inclusions of aluminium incorporated into the crystal lattice.[47]
Rhenium diboride has a very high melting signal approaching 2,400 °C and a highly anisotropic, layered crystal structure.[47] Its symmetry is either hexagonal (space group P6three mc) or orthorhombic (Cmcm) depending on the phase. There, close-packed Re layers alternate with puckered triangular boron layers forth the (001) plane. This can be seen above on the example of osmium diboride. The density of states for ReB2 has one of the everyman values among the metal borides, indicating stiff covalent bonding and loftier hardness.[46]
Attributable to the anisotropic nature of this material, the hardness depends on the crystal orientation. The (002) airplane contains the nigh covalent graphic symbol and exhibits a maximum Vickers hardness value of 40.5 GPa, while the perpendicular planes were 6% lower at 38.ane GPa. These values subtract with increased load, settling at around 28 GPa each. The nanoindentation values were found to be 36.4 GPa and 34.0 GPa for the (002) and perpendicular planes respectively. The hardness values depend on the material purity and composition – the more than boron the harder the boride – and the above values are for a Re:B ratio of approximately one.00:ane.85. Rhenium diboride also has a reported bulk modulus of 383 GPa and a shear modulus of 273 GPa.[47] [48] The hardness of rhenium diboride, and most other materials also depends on the load during the examination. The in a higher place values of about 40 GPa were all measured with an constructive load of 0.5–1 N. At such low load, the hardness values are as well overestimated for other materials, for example it exceeds 100 GPa for c-BN.[4] Other researchers, while having reproduced the high ReB2 hardness at low load, reported much lower values of 19–17 GPa at a more conventional load of 3–49 Northward, that makes ReB2 a difficult, but not a superhard cloth.[4] [13] [49]
Rhenium diboride exhibits metallic conductivity which increases as temperature decreases and tin be explained by a nonzero density of states due to the d and p overlap of rhenium and boron respectively. At this point, it is the only superhard textile with metallic behavior. The fabric as well exhibits relatively high thermal stability. Depending on the heating method, information technology will maintain its mass upwards to temperatures of 600–800 °C, with any drib being due to loss of captivated water. A small loss of mass can then exist seen at temperatures approaching 1,000 °C. It performs meliorate when a slower heat ramp is utilized. Part of this small drib at around 1,000 °C was explained by the formation of a tiresome B2Oiii blanket on the surface as boron is leached out of the solid, which serves as a protective blanket, thereby reducing additional boron loss. This can be easily dissolved by methanol to restore the textile to it native shiny state.[47] [48] [50]
Tungsten borides [edit]
The discovery of superhard tungsten tetraboride is farther evidence for the promising design arroyo of covalently bonding incompressible transition metals with boron. While WBiv was first synthesized and identified as the highest boride of tungsten in 1966,[51] it was merely recognized as an inexpensive superhard cloth in 2011.[52]
Interestingly, lower borides of tungsten such as tungsten diboride are not superhard. Higher boron content leads to college hardness considering of the increased density of brusque, covalent boron-boron and boron-metal bonds. However, researchers have been able to push WBii into the superhard regime through minority additions of other transition metals such as niobium and tantalum in the crystal construction.[53] This machinery of hardness enhancement is called solid solution strengthening and arises because atoms of unlike sizes are incorporated into the parent lattice to impede dislocation motion.
Aluminium magnesium boride [edit]
Aluminium magnesium boride or BAM is a chemical compound of aluminium, magnesium and boron. Whereas its nominal formula is AlMgB14, the chemical composition is closer to Al0.75Mg0.75B14. It is a ceramic alloy that is highly resistive to wear and has a low coefficient of sliding friction.
Other boron-rich superhard materials [edit]

Other hard boron-rich compounds include B4C and BsixO. Baggy a-B4C has a hardness of most l GPa, which is in the range of superhardness.[54] It can exist looked at as consisting of boron icosahedra-like crystals embedded in an amorphous medium. All the same, when studying the crystalline class of B4C, the hardness is only about 30 GPa. This crystalline form has the aforementioned stoichiometry as BxiiiCiii, which consists of boron icosahedra continued by boron and carbon atoms.[54] Boron suboxide (B6O) has a hardness of nigh 35 GPa. Its structure contains eight B12 icosahedra units, which are sitting at the vertices of a rhombohedral unit of measurement jail cell. In that location are two oxygen atoms located along the (111) rhombohedral direction.[55]
Nanostructured superhard materials [edit]
Nanosuperhard materials autumn into the extrinsic category of superhard materials. Because molecular defects affect the superhard backdrop of bulk materials it is obvious that the microstructure of superhard materials give the materials their unique properties. Focus on synthesizing nano superhard materials is effectually minimizing microcracks occurring within the structure through grain boundary hardening. The emptying of microcracks tin strengthen the material by three to 7 times its original strength. Grain boundary strengthening is described by the Hall-Petch equation[56]
Hither σc is the disquisitional fracture stress, d the crystallite size and σ0 and kgb are constants.
If a fabric is breakable its strength depends mainly on the resistance to forming microcracks. The critical stress which causes the growth of a microcrack of size a0 is given by a general formula[56]
Here E is the Young'southward modulus, chiliadcrevice is a constant dependent on the nature and shape of the microcrack and the stress applied and γs the surface cohesive energy.
The average hardness of a material decreases with d (crystallite size) decreasing below 10 nm. There accept been many mechanisms proposed for grain boundary sliding and hence material softening, but the details are still not understood. As well grain purlieus strengthening, much attention has been put into building microheterostructures, or nanostructures of two materials with very large differences in rubberband moduli. Heterostructures were first proposed in 1970 and contained such highly ordered sparse layers that they could not theoretically be separated past mechanical ways. These highly ordered heterostructures were believed to exist stronger than simple mixtures. This theory was confirmed with Al/Cu and Al/Ag structures. After the formation of Al/Cu and Al/Ag, the research was extended to multilayer systems including Cu/Ni, Tin/VN, W/WN, Hf/HfN and more. In all cases, decreasing the lattice period increased the hardness.[viii] One mutual form of a nanostructured material is aggregated diamond nanorods, which is harder than bulk diamond and is currently the hardest (~150 GPa) cloth known.[57]
See likewise [edit]
- Beta carbon nitride
- Borazon – Brand name of a cubic form of boron nitride (cBN)
- Iron tetraboride
- Lonsdaleite – Hexagonal lattice allotrope of carbon
- Aluminium magnesium boride
References [edit]
- ^ Wentorf, R. H.; Devries, R. C.; Bundy, F. P. (1980). "Sintered Superhard Materials". Science. 208 (4446): 873–80. doi:10.1126/science.208.4446.873. PMID 17772811. S2CID 34588568.
- ^ Fischer-Cripps, Anthony C. (2004) Nanoindentation. Springer. ISBN 0-387-22045-3. p. 198
- ^ Veprek, Due south.; Zeer, A. and Riedel, R. (2000) in Handbook of Ceramic Difficult Materials, R. Riedel (ed.). Wiley, Weinheim. ISBN iii-527-29972-6
- ^ a b c Dubrovinskaia, Northward.; Dubrovinsky, L.; Solozhenko, 5. L. (2007). "Comment on "Synthesis of Ultra-Incompressible Superhard Rhenium Diboride at Ambient Pressure"". Science. 318 (5856): 1550c. Bibcode:2007Sci...318.1550D. doi:10.1126/scientific discipline.1147650. PMID 18063772.
- ^ John, P; Polwart, N.; Troupe, C.Due east.; Wilson, J.I.B. (2002). "The oxidation of (100) textured diamond". Diamond and Related Materials. 11 (three–six): 861. Bibcode:2002DRM....xi..861J. doi:10.1016/S0925-9635(01)00673-2.
- ^ Nassau, K; Nassau, J. (1979). "The history and present status of constructed diamond". Journal of Crystal Growth. 46 (2): 157. Bibcode:1979JCrGr..46..157N. doi:10.1016/0022-0248(79)90052-6.
- ^ Tolbert, Sarah H.; Gilman, John J.; Kaner, Richard B. (2005-05-27). "Designing Superhard Materials". Science. 308 (5726): 1268–1269. doi:10.1126/science.1109830. ISSN 0036-8075. PMID 15919983. S2CID 136777087.
- ^ a b c Vepřek, Stan (1999). "The search for novel, superhard materials" (PDF). Journal of Vacuum Science and Technology A. 17 (5): 2401–2420. Bibcode:1999JVSTA..17.2401V. doi:10.1116/ane.581977.
- ^ a b c d e f g h i j Levine, Jonathan B.; Tolbert, Sarah H.; Kaner, Richard B. (2009). "Advancements in the Search for Superhard Ultra-Incompressible Metal Borides". Advanced Functional Materials. 19 (22): 3519. doi:10.1002/adfm.200901257.
- ^ a b c d e f g h i j Haines, J; Leger, JM; Bocquillon, Chiliad (2001). "Synthesis and pattern of superhard materials". Almanac Review of Materials Enquiry. 31: i–23. Bibcode:2001AnRMS..31....1H. doi:10.1146/annurev.matsci.31.i.1.
- ^ Zhao, Y.; Solozhenko, V.; Riedel, R.; Novikov, North.; Nicol, M.; Dubrovinskaia, N.; Brazhkin, V. (2004). "What does 'harder than diamond' mean?". Nature Materials. 3 (9): 576–577. doi:10.1038/nmat1196. ISSN 1476-4660. PMID 15343282. S2CID 39507507.
- ^ a b c d due east Solozhenko, 5. L.; Kurakevych, Oleksandr O.; Andrault, Denis; Le Godec, Yann; Mezouar, Mohamed (2009). "Ultimate Metastable Solubility of Boron in Diamond: Synthesis of Superhard Diamondlike BCfive" (PDF). Phys. Rev. Lett. 102 (1): 015506. Bibcode:2009PhRvL.102a5506S. doi:10.1103/PhysRevLett.102.015506. PMID 19257210.
- ^ a b Qin, Jiaqian; He, Duanwei; Wang, Jianghua; Fang, Leiming; Lei, Li; Li, Yongjun; Hu, Juan; Kou, Zili; Bi, Yan (2008). "Is Rhenium Diboride a Superhard Material?". Avant-garde Materials. 20 (24): 4780. doi:10.1002/adma.200801471.
- ^ Xie, Zhilin (2017). "Aluminum magnesium boride: synthesis, sintering, and microstructure". Advances in Applied Ceramics. 116 (6): 341–347. doi:10.1080/17436753.2017.1317116. S2CID 135978454.
- ^ Lee, J. & Novikov N. 5. (2005). Innovative superhard materials and sustainable coatings for advanced manufacturing. Springer. p. 102. ISBN978-0-8493-3512-9.
- ^ Marinescu, I. D.; Tönshoff, H. K. & Inasaki, I. (2000). Handbook of ceramic grinding and polishing. William Andrew. p. 21. ISBN978-0-8155-1424-iv.
- ^ Kutz, Myer (2002). Handbook of materials selection. John Wiley and Sons. ISBN 0-471-35924-6 p. 384
- ^ Wei, Lanhua; Kuo, P.; Thomas, R.; Anthony, T.; Banholzer, Westward. (1993). "Thermal conductivity of isotopically modified single crystal diamond". Physical Review Letters. 70 (24): 3764–3767. Bibcode:1993PhRvL..70.3764W. doi:10.1103/PhysRevLett.70.3764. PMID 10053956.
- ^ Walker, J (1979). "Optical absorption and luminescence in diamond". Reports on Progress in Physics. 42 (10): 1605–1659. Bibcode:1979RPPh...42.1605W. CiteSeerX10.1.one.467.443. doi:ten.1088/0034-4885/42/10/001.
- ^ Barnard, A. South. (2000) The diamond formula: diamond synthesis—a gemmological perspective. Butterworth-Heinemann. ISBN 0-7506-4244-0
- ^ Liander, H. (1955). "Artificial diamonds". ASEA Periodical. 28: 97.
- ^ "Man-Fabricated Diamonds". Chemical & Engineering News. 33 (eight): 718. 1955. doi:10.1021/cen-v033n008.p718.
- ^ a b Wentorf, R. H. (1957). "Cubic Form of Boron Nitride". The Journal of Chemical Physics. 26 (4): 956. Bibcode:1957JChPh..26..956W. doi:10.1063/1.1745964.
- ^ a b Lonsdale, Kathleen (1962). "Further Comments on Attempts by H. Moissan, J. B. Hannay and Sir Charles Parsons to Make Diamonds in the Laboratory". Nature. 196 (4850): 104–106. Bibcode:1962Natur.196..104L. doi:10.1038/196104a0. S2CID 29498398.
- ^ Catledge, Shane A.; Vohra, Yogesh K. (1999). "Effect of nitrogen addition on the microstructure and mechanical properties of diamond films grown using loftier-methane concentrations". Journal of Practical Physics. 86 (one): 698. Bibcode:1999JAP....86..698C. doi:ten.1063/one.370787.
- ^ Ekimov, E. A.; Sidorov, V. A.; Bauer, Due east. D.; Mel'nik, Due north. Due north.; Curro, N. J.; Thompson, J. D.; Stishov, S. M. (2004). "Superconductivity in diamond". Nature. 428 (6982): 542–5. arXiv:cond-mat/0404156. Bibcode:2004Natur.428..542E. doi:10.1038/nature02449. PMID 15057827. S2CID 4423950.
- ^ Tian, Yongjun; Liu, Zhongyuan; He, Julong; Wen, Bin; Zhao, Zhisheng; Wang, Yanbin; Ma, Yanming; Hu, Wentao; Xu, Bo (2014). "Nanotwinned diamond with unprecedented hardness and stability". Nature. 510 (7504): 250–253. Bibcode:2014Natur.510..250H. doi:ten.1038/nature13381. ISSN 1476-4687. PMID 24919919. S2CID 4466193.
- ^ Zhang, Shuangshuang (2021). "Discovery of carbon-based strongest and hardest amorphous material". National Science Review. arXiv:2011.14819. doi:10.1093/nsr/nwab140.
- ^ a b c Greim, Jochen; Schwetz, Karl A. (2005). "Boron Carbide, Boron Nitride, and Metallic Borides". Boron Carbide, Boron Nitride, and Metal Borides, in Ullmann'south Encyclopedia of Industrial Chemistry. Wiley-VCH: Weinheim. doi:ten.1002/14356007.a04_295.pub2. ISBN978-3527306732.
- ^ Bocquillon, Thousand.; Loriers-Susse, C.; Loriers, J. (1993). "Synthesis of cubic boron nitride using Mg and pure or Yard'-doped Li3N, CathreeN2 and Mg3N2 with Chiliad'=Al, B, Si, Ti". Journal of Materials Science. 28 (13): 3547. Bibcode:1993JMatS..28.3547B. doi:10.1007/BF01159836. S2CID 96651315.
- ^ Leichtfried, G.; et al. (2002). "xiii.v Properties of diamond and cubic boron nitride". In Beiss, P. (ed.). Landolt-Börnstein – Group Viii Advanced Materials and Technologies: Powder Metallurgy Data. Refractory, Hard and Intermetallic Materials. Landolt-Börnstein - Group VIII Advanced Materials and Technologies. Vol. 2A2. Berlin: Springer. pp. 118–139. doi:10.1007/b83029. ISBN978-3-540-42961-half-dozen.
- ^ El Khakani, M. A.; Chaker, M. (1993). "Physical properties of the x-ray membrane materials". Journal of Vacuum Science and Technology B. 11 (6): 2930. Bibcode:1993JVSTB..11.2930E. doi:10.1116/1.586563.
- ^ Wilke, K.T. and Bohm, J. (1988) Kristallzüchtung, Verlag Harri Deutsch, Frankfurt.
- ^ Liu, A. Y.; Cohen, One thousand. 50. (1989). "Prediction of New Low Compressibility Solids". Science. 245 (4920): 841–2. Bibcode:1989Sci...245..841L. doi:x.1126/science.245.4920.841. PMID 17773359. S2CID 39596885.
- ^ Teter, D. M.; Hemley, R. J. (1996). "Low-Compressibility Carbon Nitrides". Scientific discipline. 271 (5245): 53. Bibcode:1996Sci...271...53T. doi:10.1126/science.271.5245.53. S2CID 220100338.
- ^ Yin, Long-Wei; Li, Mu-Sen; Liu, Yu-Xian; Sui, Jin-Ling; Wang, Jing-Min (2003). "Synthesis of beta carbon nitride nanosized crystal through mechanochemical reaction". Journal of Physics: Condensed Matter. 15 (2): 309. Bibcode:2003JPCM...xv..309Y. doi:10.1088/0953-8984/15/2/330.
- ^ Badzian, R.; Niemyski, T. and Olkusnik, Eastward. (1972) in Proceedings of the 3rd International Conference on Chemic Vapor Degradation, Salt Lake City, April 1972, F. A. Galski (ed.), p. 747
- ^ Solozhenko, Vladimir L.; Andrault, Denis; Fiquet, Guillaume; Mezouar, Mohamed; Rubie, David C. (2001). "Synthesis of superhard cubic BC2N". Practical Physics Letters. 78 (10): 1385. Bibcode:2001ApPhL..78.1385S. doi:x.1063/i.1337623.
- ^ Solozhenko, Five; Gregoryanz, E (2005). "Synthesis of superhard materials". Materials Today. 8 (11): 44. doi:10.1016/S1369-7021(05)71159-7.
- ^ Akopov, Georgiy; Yeung, Michael T.; Kaner, Richard B. (June 2017). "Rediscovering the Crystal Chemical science of Borides". Avant-garde Materials. 29 (21): 1604506. doi:ten.1002/adma.201604506. PMID 28323358.
- ^ Mohammadi, R.; Lech, A. T.; Xie, M.; Weaver, B. Due east.; Yeung, Thou. T.; Tolbert, S. H.; Kaner, R. B. (2011). "Tungsten tetraboride, an inexpensive superhard fabric". Proceedings of the National University of Sciences. 108 (27): 10958–62. Bibcode:2011PNAS..10810958M. doi:10.1073/pnas.1102636108. PMC3131357. PMID 21690363.
- ^ Mohammadi, R.; Xie, Grand.; Lech, A. T.; Turner, C. L.; Kavner, A.; Tolbert, S. H.; Kaner, R. B. (2012). "Toward Inexpensive Superhard Materials: Tungsten Tetraboride-Based Solid Solutions". Journal of the American Chemic Society. 134 (51): 20660–8. doi:x.1021/ja308219r. PMID 23171079.
- ^ Robinson, Paul J.; Liu, Gaoxiang; Ciborowski, Sandra; Martinez-Martinez, Chalynette; Chamorro, Juan R.; Zhang, Xinxing; McQueen, Tyrel 1000.; Bowen, Kit H.; Alexandrova, Anastassia N. (16 Nov 2017). "Mystery of 3 Borides: Differential Metal–Boron Bonding Governing Superhard Structures". Chemistry of Materials. 29 (23): 9892–9896. doi:10.1021/acs.chemmater.7b04378.
- ^ Cumberland, Robert W.; Weinberger, Michelle B.; Gilman, John J.; Clark, Simon One thousand.; Tolbert, Sarah H.; Kaner, Richard B. (2005). "Osmium Diboride, An Ultra-Incompressible, Hard Material". Journal of the American Chemical Lodge. 127 (20): 7264–5. doi:10.1021/ja043806y. PMID 15898746.
- ^ Chen, Z.; Xiang, H.; Yang, Jinlong; Hou, J.; Zhu, Qingshi (2006). "Structural and electronic properties of OsB2: A hard metallic material". Physical Review B. 74 (one): 12102. arXiv:cond-mat/0508506. Bibcode:2006PhRvB..74a2102C. doi:ten.1103/PhysRevB.74.012102.
- ^ a b c Gou, Huiyang; Wang, Zhibin; Zhang, Jingwu; Yan, Shuting; Gao, Faming (2009). "Structural Stability and Elastic and Electronic Properties of Rhenium Borides: Showtime Principle Investigations". Inorganic Chemistry. 48 (two): 581–7. doi:10.1021/ic8019606. PMID 19072687.
- ^ a b c d Levine, Jonathan B.; Nguyen, Sandy L.; Rasool, Haider I.; Wright, Jeffrey A.; Brown, Stuart East.; Kaner, Richard B. (2008). "Preparation and Backdrop of Metallic, Superhard Rhenium Diboride Crystals". Journal of the American Chemical Gild. 130 (l): 16953–8. doi:10.1021/ja804989q. PMID 19053446.
- ^ a b Levine, J.B.; Betts, J.B.; Garrett, J.D.; Guo, S.Q.; Eng, J.T.; Migliori, A.; Kaner, R.B. (2010). "Total elastic tensor of a crystal of the superhard compound ReBii". Acta Materialia. 58 (5): 1530. Bibcode:2010AcMat..58.1530L. doi:ten.1016/j.actamat.2009.ten.060.
- ^ Gu, Qinfen; Krauss, Guenter; Steurer, Walter (2008). "ChemInform Abstract: Transition Metal Borides: Superhard versus Ultra-Incompressible". ChemInform. 39 (50). doi:ten.1002/chin.200850007.
- ^ Šimůnek, A (2009). "Anisotropy of hardness from kickoff principles: The cases of ReBii and OsB2". Physical Review B. 80 (vi): 60103. Bibcode:2009PhRvB..80f0103S. doi:ten.1103/PhysRevB.80.060103.
- ^ Krug, One thousand. P.; Romans, P. A. (1966-02-10). "Limerick and crystallographic information for the highest boride of tungsten". Acta Crystallographica. twenty (two): 313–315. doi:10.1107/S0365110X6600063X. ISSN 0365-110X.
- ^ Kaner, Richard B.; Tolbert, Sarah H.; Yeung, Michael T.; Weaver, Beth E.; Xie, Miao; Lech, Andrew T.; Mohammadi, Reza (2011-07-05). "Tungsten tetraboride, an cheap superhard fabric". Proceedings of the National Academy of Sciences. 108 (27): 10958–10962. Bibcode:2011PNAS..10810958M. doi:10.1073/pnas.1102636108. ISSN 0027-8424. PMC3131357. PMID 21690363.
- ^ Pangilinan, Lisa E.; Turner, Christopher L.; Akopov, Georgiy; Anderson, Mackenzie; Mohammadi, Reza; Kaner, Richard B. (2018-12-17). "Superhard Tungsten Diboride-Based Solid Solutions". Inorganic Chemical science. 57 (24): 15305–15313. doi:ten.1021/acs.inorgchem.8b02620. ISSN 0020-1669. PMID 30516362.
- ^ a b Ulrich, S; Ehrhardt, H.; Schwan, J.; Samlenski, R.; Brenn, R. (1998). "Subplantation effect in magnetron sputtered superhard boron carbide sparse films". Diamond and Related Materials. 7 (6): 835. Bibcode:1998DRM.....7..835U. doi:10.1016/S0925-9635(97)00306-3.
- ^ Hubert, Hervé; Garvie, Laurence A. J.; Devouard, Bertrand; Buseck, Peter R.; Petuskey, William T.; McMillan, Paul F. (1998). "Loftier-Force per unit area, High-Temperature Synthesis and Characterization of Boron Suboxide (B6O)". Chemistry of Materials. 10 (6): 1530. doi:x.1021/cm970433+.
- ^ a b Bouchaud, Elisabeth; Jeulin, Dominique and Prioul, Claude (2001) Physical aspects of fracture. Springer. ISBN 0-7923-7147-X. p. 23
- ^ Blank, Five; Popov, K.; Pivovarov, Chiliad.; Lvova, North.; Gogolinsky, Thousand.; Reshetov, V. (1998). "Ultrahard and superhard phases of fullerite C60: Comparison with diamond on hardness and wear" (PDF). Diamond and Related Materials. 7 (2–5): 427. Bibcode:1998DRM.....7..427B. CiteSeerX10.1.one.520.7265. doi:10.1016/S0925-9635(97)00232-X. Archived from the original on July 21, 2011.
{{cite journal}}: CS1 maint: unfit URL (link)
Source: https://en.wikipedia.org/wiki/Superhard_material#:~:text=Diamond%20is%20the%20hardest%20known,practical%20applications%20of%20this%20material.


0 Response to "what is the hardest thing in the world to cut"
Post a Comment